Medidata eConsent
Empowering Patients Right From the Start
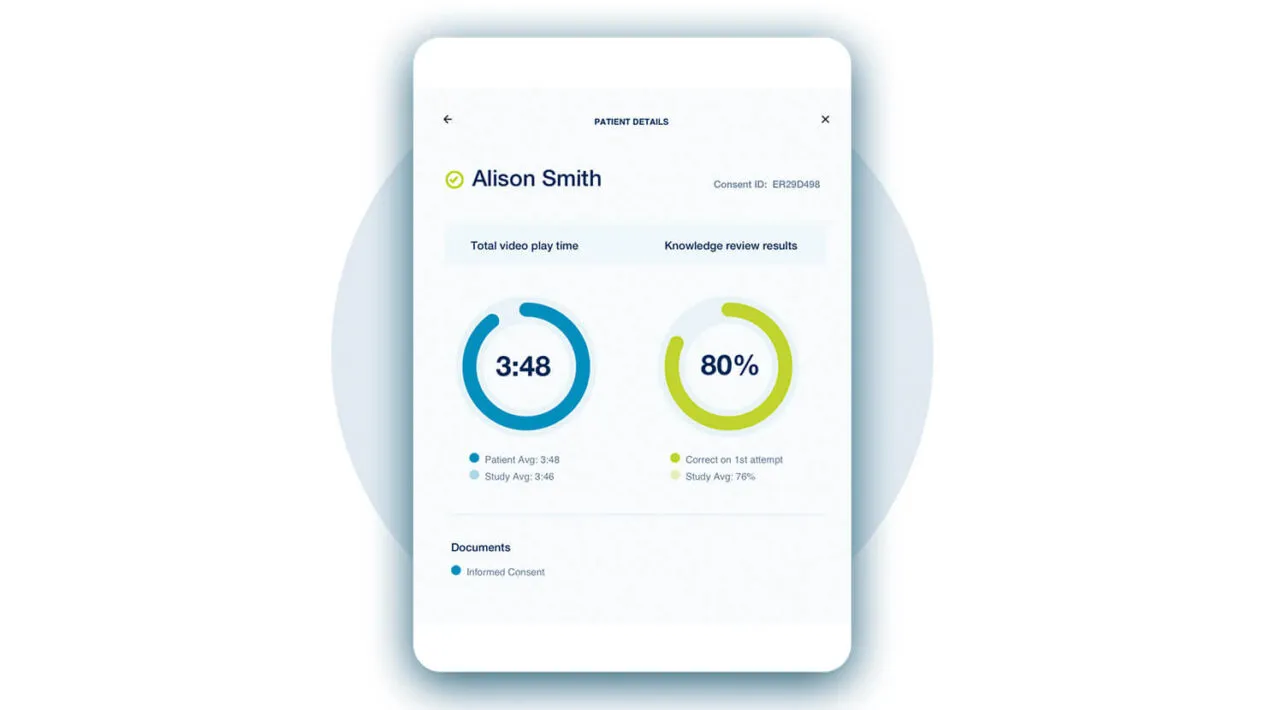
What is eConsent for clinical trials? eConsent allows patients to understand trial objectives while providing consent through multimedia technology onsite or remotely.
Medidata’s eConsent is an innovative, regulatory-compliant, patient-friendly, electronic consent system for clinical trials. Whether onsite or remote, Medidata eConsent automates the patient enrollment process and onboards patients directly into Rave EDC, improving overall consent tracking management, reducing informed consent errors, and easing the administrative burden for sites and study teams. It also enhances the patient experience with easy-to-understand clinical trial information while improving patient compliance and boosting patient engagement.
The Value of Medidata eConsent
Create a Better Patient Experience
Medidata eConsent advances patient compliance and retention while enabling an increased understanding of study objectives, risks, benefits, and responsibilities. Our dedicated Patient Cloud Helpdesk optimizes the site and patient experience when and where you need it most.

Unified Platform Approach
Medidata eConsent comes unified with the Medidata Platform, the platform already used by the majority of the clinical trials in the world. While our competitors may be compatible with the Medidata Platform, integration is not simplification.

Path to Hybrid & Fully Decentralized Studies
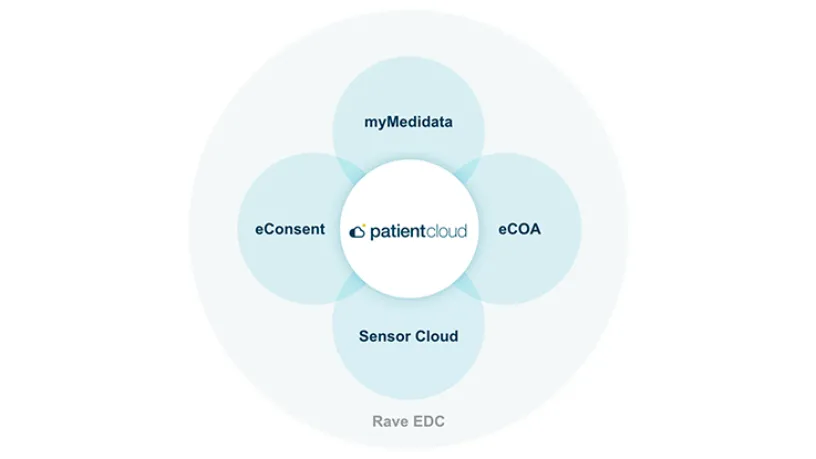
Part of the Patient Cloud suite of solutions, Medidata’s eConsent easily captures patient consent regardless of location while supporting 100% BYOD for added flexibility.
Key Features of Medidata eConsent

Multiple Ways to Engage
Built with the ultimate flexibility in mind, patients in clinical trials can choose to provide consent at the site or remotely depending upon the design of the study.

Integrated with Rave EDC
Medidata eConsent comes unified with the Medidata Platform, the platform already used by the majority of the clinical trials in the world. While our competitors may be compatible with the Medidata Platform, integration is not simplification.

User-Friendly Configuration Tools
Our clinical trial technology eliminates the need for developer support, customization, html conversion, and duplicate effort which helps to decrease eConsent study and site set-up timelines from months to weeks.
Related Solutions
Learn More

The Use of Electronic Informed Consent (eConsent) in a Blood Collection Study
A specialty division of a top ten pharma was preparing for an upcoming blood collection study, looking to recruit 6,000 patients spanning ten sites.
Moving away from their paper process, Medidata eConsent modernized the clinical trial consenting process for patients, sites, and sponsors. Learn more about how Medidata eConsent improved patient comprehension and reduced site workload.

Empowering Patients Right from the Start
Medidata’s eConsent is an innovative, patient-friendly, electronic informed consent and patient enrollment system for clinical trials. Built with the ultimate flexibility in mind, patients can choose to provide consent at the site or remotely depending upon the design of the study.

Electronic Informed Consent in Clinical Research
This white paper provides an overview of findings from the Medidata eConsent study to understand the regulatory positions, adoption, and the variability regarding eConsent globally.