Managing Clinical Trial Data in a Fast-paced, Complex Environment

The world of clinical data management is rapidly changing, and so too are the skills and processes associated with today’s data managers. New data sources bring in higher volumes of data at faster speeds, giving teams more insights around patients than ever before. But data managers continue to face challenges with regards to data collection and data review.
Below are the most common issues that data managers experience, according to Medidata’s 2022 Survey of Buyers and Users of Clinical Data Management Tools.
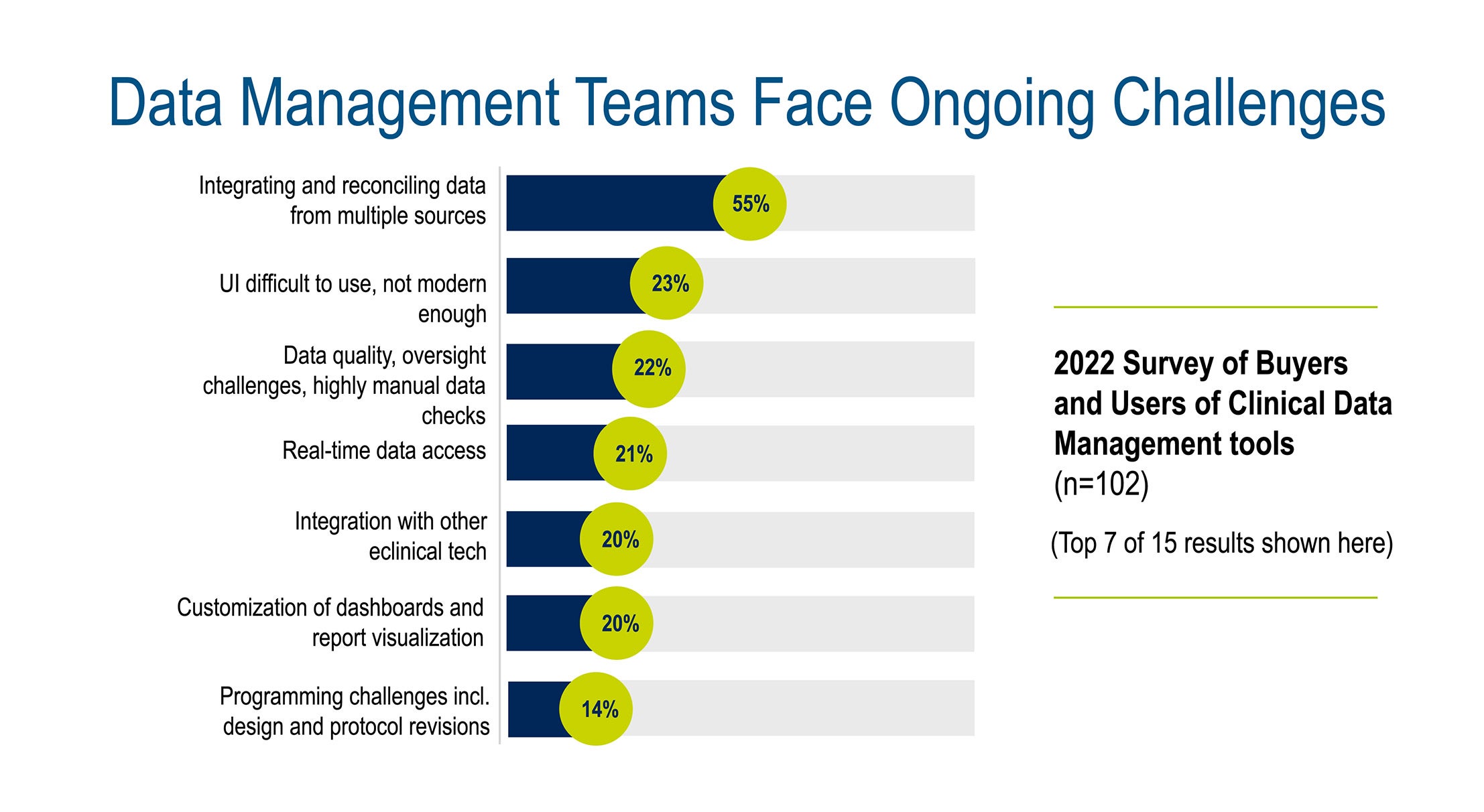
The need for multiple systems creates silos that require programming to aggregate and reconcile data. Yesterday’s traditional data management approaches no longer scale due to the high velocity and sheer amount of data across numerous sources. And manually reviewing, cleaning, and locking data is an inefficient and exhaustive process for data management teams.
Medidata NEXT New York 2022 brought together the industry’s most prominent figures and organizations to discuss these obstacles, as well as exciting solutions to streamline clinical data management processes.
Read on for highlights from three popular sessions.
1) What’s New and Next in Medidata Rave Data Management
This session covered exciting developments and updates for Medidata’s Rave Data Management solutions in 2022 and 2023. Rave Data Management is focusing on five main areas of innovation, starting with protocol-driven study design. The new Medidata Designer tool will elevate each study design above any data ingestion or data acquisition mechanism. This tool provides a single source of truth for study designs based on the schedule of activities and the datasets to be collected in each activity. Configuration of specific design elements such as eCRFs and eCOA questionnaires is then layered on top of the common data definitions to deliver working systems.
The next area of focus is aggregated data review. Medidata’s Data Reviewer capability enables aggregated review of data from multiple sources without the need for programming. For data originating from Rave EDC (electronic data capture), queries are automatically generated and posted back to the correct patient, form and field, eliminating the need for manual and time-consuming posting of queries. In 2023, these capabilities will be extended with automated data reconciliation for adverse events, concomitant medications, and medical history.
The third area is EHR (electronic health records) and bedside capture. In addition to the EHR/EDC integration through Rave Companion, Medidata is also developing a native app that will allow for bedside data capture in a streamlined workflow to only capture what’s necessary. Home health nurses will be able to go directly to a patient’s home and collect that data.
The fourth area, automated medical coding, introduced Rave Coder+. This offering is trained with over 60 million coding decisions to streamline workflows, as well as the first Chinese coding solution unified with a leading EDC system.
The final area of focus is user experience and clinical analytics. Detailed dashboards will be available to elevate tasks and present critical information to data managers and clinical operations colleagues as soon as they log into the platform. These dashboards will be customizable to better visualize and analyze data.
2) The Evolution of eSource and the Revolution in Clinical Data Management
Representatives from Medidata, Merck, Moderna, and ICON sat down at NEXT New York 2022 to discuss how the evolution of eSource has changed data management today. Data capture is evolving past manual capture (paper consent, diaries, etc.) to automated capture (health records, medical imaging, etc.) and direct capture from patients (eCOA, eConsent, wearable sensors, etc.). This evolution also brings increases in data volume, velocity, variety, veracity, and value.
Clinical data management teams require process changes to keep up with the shifting landscape. Evan Hughes, Senior Director and Head of Americas Data Management at ICON, noted the importance of identifying the purpose of the data being captured and how that data will be used. Properly defining the purpose of data upfront in study protocols and its relation to primary or secondary exploratory endpoints saves time when collecting and cleaning data.
Prasann Mehta, Director of IRT & eCOA Services at Merck, highlighted the need to examine the end-to-end processes in place. Cross-functional areas, like CRAs and site processes, are also impacted by process changes; it’s imperative to bring the community together for every change.
Laurie Callen, Senior Director of Clinical Data Management at Moderna, emphasized that the traditional process of manually reviewing data line by line is no longer feasible in a world of 40,000-patient studies and billions of data points. From a trend perspective, leveraging advanced analytics and risk-based monitoring approaches to understand the stories within the data can help determine when to take action as early as possible within a clinical trial.
3) Solving the EHR to EDC Challenge
Medidata and Syneos Health discussed the obstacles surrounding EHR to EDC integration. Research coordinators at trial sites are increasingly frustrated about the sheer volume of data they are asked to manually re-enter into EDC systems that’s already been captured in their EHR systems. Known as “swivel chair integration”, this time-consuming process leads to more errors, which in turn leads to more queries needing to be raised and resolved.
Solving this problem has proven very difficult. IT systems need to be installed, data needs to be mapped, and data agreements need to be negotiated between the site, vendor, and sponsor. Many sites, like community health centers, don’t have commercial EMR (electronic medical records) or EHR solutions and instead use homegrown systems.
Clinical research sites need scalable solutions that reduce the time it takes to get set up for clinical trials. They need to reduce overall monitoring activities and costs, while automating how people are capturing data from the EHR system into the EDC system and providing traceability into that data. And they need a solution that works for everybody depending on the level of connectivity and technology at each particular site.
The newly-released Medidata Rave Companion is designed to make EHR to EDC integration seamless. This tool streamlines the process to help site staff spend less time on tasks and more time on patients.
Watch the Full Sessions From NEXT New York 2022
The leading minds in life sciences came together at NEXT New York 2022 to map the future of clinical data management. Access the full sessions below and discover how Medidata is solving today’s clinical data management challenges.
Explore Related Articles
Contact Us


