Continuous Innovation—Why the Medidata Platform is a Leader Among Clinical Data and Analytics Platforms

Driving continuous innovation and the user experience is at the heart of Medidata’s DNA. In July 2023, The Everest Group positioned Medidata as an industry leader in their Life Sciences Clinical Data and Analytics (D&A) Platforms PEAK Matrix® Assessment. This recognition is the latest in a long list of accolades. It stems from the innovative work Medidata continues to do to build the industry’s leading patient- and site-centric clinical research platform that empowers our customers to deliver smarter treatments and better patient outcomes.
Today, Medidata provides best-in-class real-time data availability and ease of access to extract data with:
- Easier and faster (1) data entry at sites and (2) acquisition of data from patients
- Streamlined data quality workflows
- Comprehensive reporting (both out-of-the-box and custom)
- Open web services API (application programming interface)
Medidata provides, enables, and supports data capture and management from multiple sources and visibility of the appropriate data within Rave EDC for research sites, sponsors, and CROs through:
- eConsent
- eCOA/ePRO
- Sensors
- Imaging
- EHR (electronic health record) data via Rave Companion
- Non-Medidata data sources (e.g., sponsor/CRO/3rd-party systems)
For a complete data quality solution, Medidata supports RBQM (risk-based quality management and Patient Data Surveillance) in our Medidata Detect solution as standard.
Medidata is highly rated in industry analyst reports, peer reviews, and general reviews across the board, and our customers’ testimonials speak for themselves:
“So, with all the data in Medidata’s one platform, it’s great, it’s easy...we’re not downloading from another platform and comparing. It’s all there, and it’s easier to integrate.”
– Cathy Hult, Director of Data Management, PROMETRIKA
“The best qualities of Rave EDC are how user-friendly [it is] for the sites to enter data..., how the query management is very efficient, and [the ease of] getting the data out.”
– Vijay Chundru, Senior Director, EDC Programming Team, Global Clinical Data Operations, Jazz Pharmaceuticals
We live in a constantly changing world, and the complex clinical trials landscape continues to evolve rapidly. Significant market trends that reflect this include the following:
- The growing complexity of data in clinical trials requires the use of advanced technologies to manage and analyze an increasing volume of generated data
- Trial designs have become more complex: the use of adaptive study designs, more frequent master protocol trials, the incorporation of real-world data, and the increased focus on more extensive and more diverse patient populations
- The concentration of clinical trials in specific therapeutic areas, such as oncology, leading to novel immunotherapeutic approaches (CAR-T)
- Precision medicine: the shift in trial designs towards targeted studies with a greater focus on biomarkers and personalized outcomes
In addition to the above, technology trends that are positively impacting the clinical trials landscape include:
- Increased adoption of low-code environments, which improve productivity and compliance while driving down the total cost of ownership
- The drive for interoperability and open standards, which are crucial to clinical and patient-care solutions as they evolve into a data, services, and supplier ecosystem
- The use of data streaming technologies that transform clinical data management while facilitating the growth of decentralized clinical trials
- The implementation of Al/ML technologies in clinical data management for improved efficiency and reduced time to database lock
Medidata’s part in driving these trends has been significant. Building on the current Medidata Platform, experience, and customer/industry feedback, what does an intelligent clinical data acquisition, management, and analytics platform look like?
Medidata’s Next-Generation Clinical Data Platform
The Medidata Platform is used in more commercial clinical trials worldwide than any other clinical research technology, powering 33,000+ trials and involving over 9 million patients. Building on this foundation, Medidata’s next-generation platform accelerates data flow from protocol to submission (Fig. 1).
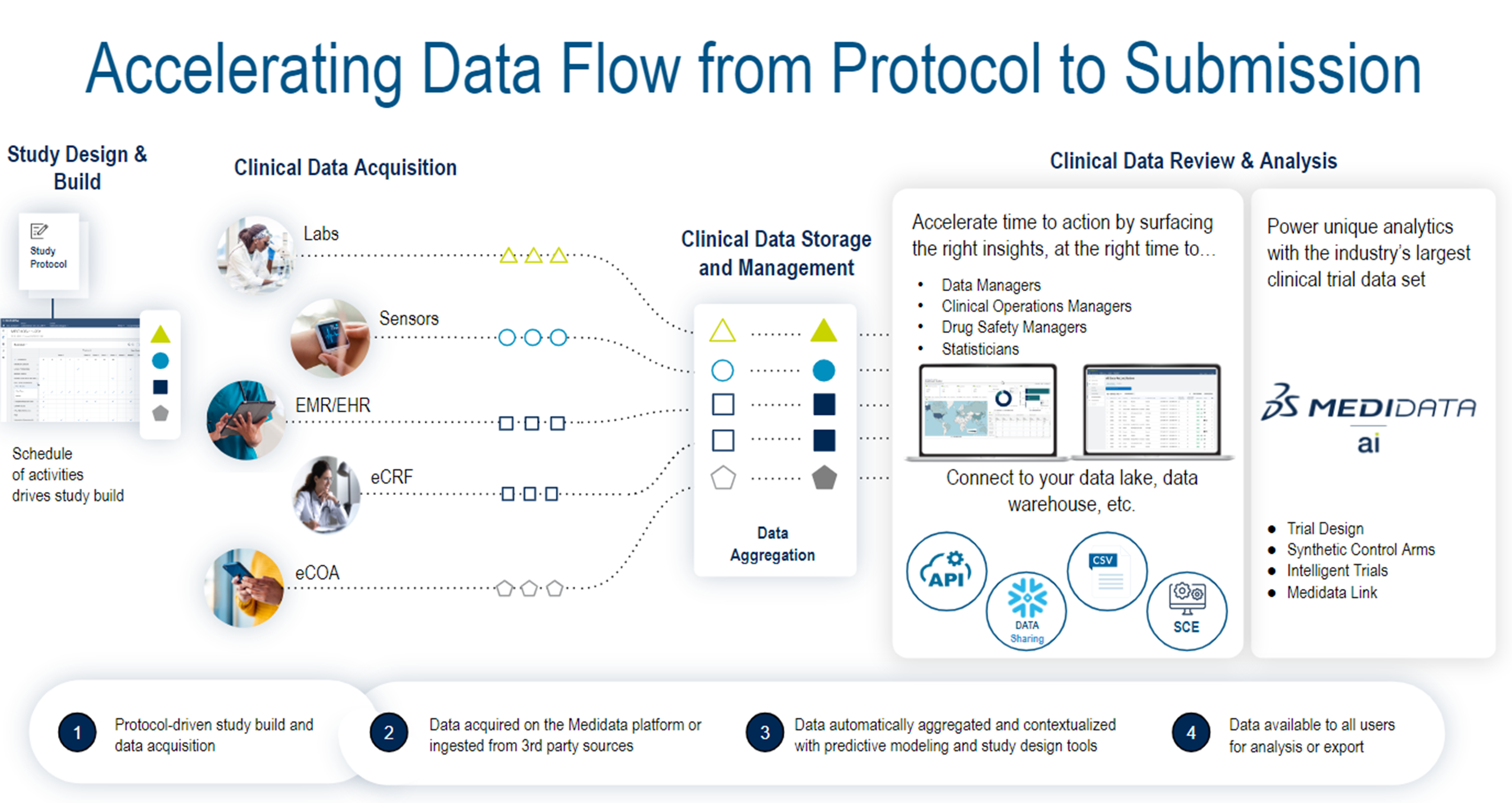
Fig. 1: The next-generation, standards-based data infrastructure underpinning the Medidata Platform.
There are three core areas: Clinical data acquisition from multiple sources, clinical data management, and clinical data review and analysis.
Clinical Data Acquisition from Multiple Sources
The Medidata Platform provides integrated, multi-source data capture solutions from Medidata and non-Medidata data sources, including labs, sensors, EHR, eCRF, eCOA/ePRO, and imaging. Clients can adopt these embedded data acquisition capabilities and ingest such data from their systems or third-party vendors into the platform for study teams to review and consume. Data collected on Medidata solutions or acquired externally are connected and surfaced in a single location without manual data re-entry, reconciliation, or uploads. Data in the platform is automatically available for real-time reporting, data review, anomaly detection, and analytics.
Clinical Data Management
Medidata’s data storage and management is robust and flexible, accommodating various operating models from users. Moving forward, we are significantly enhancing the platform with innovative and advanced capabilities:
- Predictive coding trained on over 60 million historic coding decisions
- A single, unified design environment for all metadata across each trial
- Intuitive data ingestion, data discovery, mapping/enrichment, and a data transformation tool for non-technical users
The platform is powered by next-gen architecture, which is highly adaptive to different workflows/operating models, interoperable, and provides end-to-end traceability, including:
- Graph-based biomedical concepts, which are data model agnostic and embedded into the platform
- Data streaming technology for automated real-time, streaming data, end-to-end
- Robust data governance and catalog with record-level control
Clinical Data Review and Analysis
Medidata Detect’s comprehensive data review and analysis capabilities leverage automation and advanced analytics to help users ensure data integrity and trial quality across data sources. The platform’s modules provide clean, integrated patient data from many sources and deliver simplified patient reviews, as well as real-time site and study performance monitoring via Key Risk Indicators (KRIs), Quality Tolerance Limits (QTLs), and anomaly and fraud detection, resulting in faster time to database lock. The system leverages several next-gen technologies, including automated query management, Al-driven data reconciliation with human-in-the-loop adjudication, and unsupervised ML-driven anomaly detection.
Medidata Al offers access to unparalleled clinical data, advanced analytics capabilities, and industry expertise founded on extensive real-world data sources and support of over 33,000 trials. Our innovative data science offerings support trial design, advanced study feasibility, diversity in clinical trials, and operational analytics intelligence.
Unifying Patients, Sites, Clinical Operations, Clinical Data Management, and Other Clinical Research Stakeholders to Accelerate Time to Data
The Medidata Platform combines patient and site data acquisition experiences with study and data management workflows to empower sponsors and CROs to get high-quality data faster and bring treatments to patients in need sooner.
Learn more about Medidata’s next-generation clinical data platform by attending our flagship event, NEXT New York (Nov 7-8). Not able to make it to New York? Then look out for us coming to a city near you in 2024!
Explore Related Articles
Contact Us

