Archive: 2016
TransCelerate and Medidata Explore SDV Effectiveness in Upcoming Webinar
It’s no secret that clinical trial complexity and drug development costs are at an all-time high. With more than 30 percent of trial budgets allocated to site monitoring costs—and more than half of that being spent on source document verification (SDV) activities¹—the idea of implementing a risk-based monitoring (RBM) program to rid ourselves of the traditional 100-percent-SDV approach has the life sciences industry buzzing. Read More

The Future of eClinical Solutions and Integrating Systems and Data
Robert Musterer is president of ER Squared, an eClinical consulting company partnering with Zifo Technologies, and presented at the recent Medidata Symposium. In this video interview conclusion, Medidata’s Claribel Pichardo chats with Robert about the evolution of eClinical systems from EDC to a portfolio of solutions supporting the clinical trial process. Read More
How New Technologies Can Change the Way We Manage Clinical Trial Processes
We recently sat down with Robert Musterer, president of ER Squared, an eClinical consulting company partnering with Zifo Technologies. Robert presented at the Medidata Symposium on "Overcoming Inertia for Improved Clinical Trial Designs," a topic he covered in this recent Geeks Talk Clinical blog post. Read More
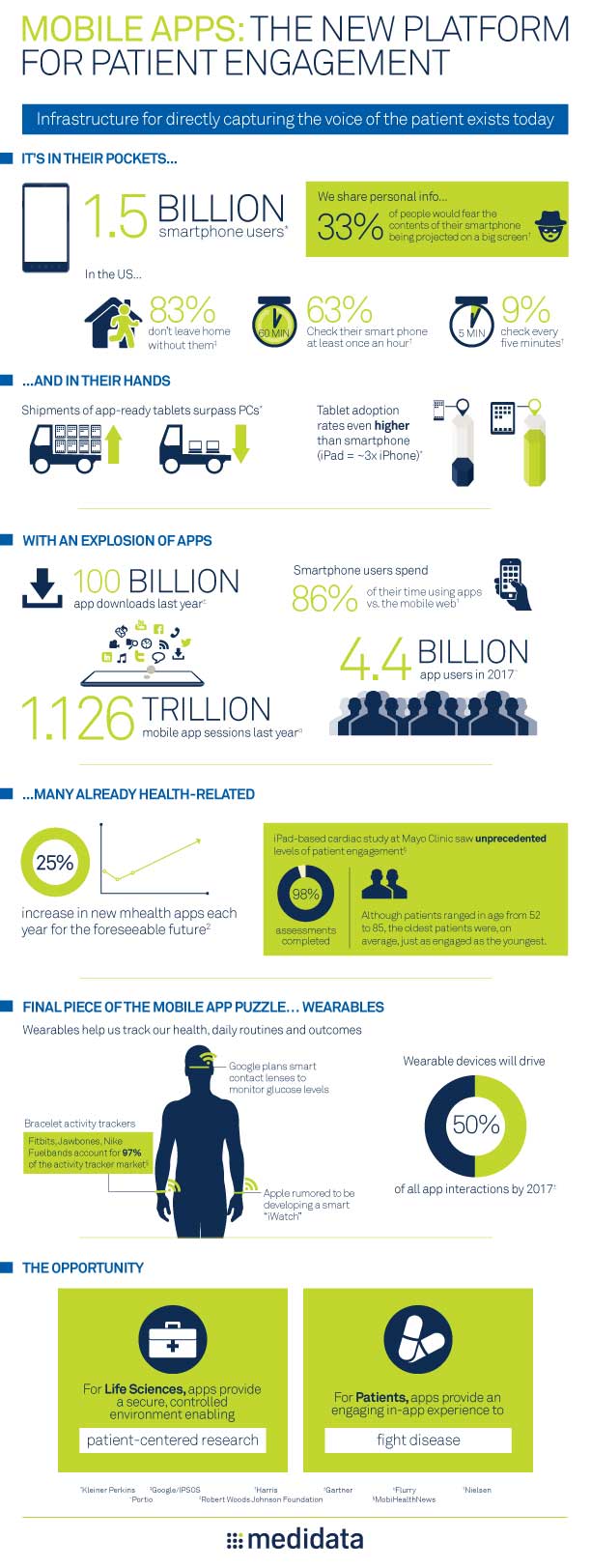
Mobile Apps: The New Platform for Patient Engagement
Capturing the voice of the patient has never been more critical and the life sciences industry now has the potential to harness the passion of patients to fight disease. Read More
Forbes Gathers Industry Leaders to Share Thoughts on “Empowering the Patient Revolution”
This week, more than 200 leaders from across the industry will convene at the Forbes Healthcare Summit to discuss the changes newly empowered patients are bringing to the healthcare landscape. Medidata is again sponsoring the summit because it is exactly the type of collaborative environment that is needed if healthcare and research are going to break out of current models. Read More

Challenges in Penetrating a Paper-Based Market with Technology-Based Solutions
The usage of electronic data capture (EDC) and other technology-based solutions in clinical trials—e.g., interactive web-response systems (IWRS), clinical trial management systems (CTMS) and electronic patient-reported outcome (ePRO) tools—has shown a substantial growth over the last decade. However in Israel, the adoption rate of EDC by locally-based companies has not increased, and many are still using paper-based solutions for their trials. Read More

Women Scientists – Are You Thinking About Your Own Personal Development?
To excel in a scientific career with an advanced degree, a rigorous and arduous training regimen is required. During the graduate and post-doctoral training phases of this career path, one spends much of their time focused on advancing projects, analyzing data, writing manuscripts and preparing seminars. Read More

