Imaging for CROs
The Core Lab-Agnostic Data Management Solution for CROs
The global clinical trials imaging market is expected to double to $2 billion by 2030.1
CROs have the opportunity to gain control and power more sponsors by adopting a core lab-agnostic imaging data management solution.
Rave Imaging enables CROs to secure management and visibility of imaging data while simplifying end-to-end workflows across sites, sponsors, and core labs.
1. 2022 Report by Grand View Research, Inc.

Rethink your Imaging Strategy
Reduce Overall Costs
Rave Imaging helps CROs regain management and financial control of imaging by enabling their sponsors to reduce manual steps, data reconciliation, rework and out-of-pocket expenses, image prep time, and radiologist assessment read time.
Scale and Customize
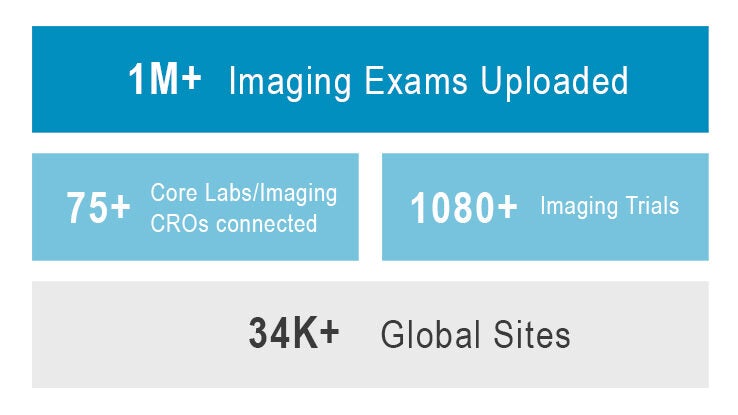
By adopting a single core lab-agnostic data management solution for all trials, CROs help sponsors regain control over their critical images and accelerate their timelines while ensuring compliance.
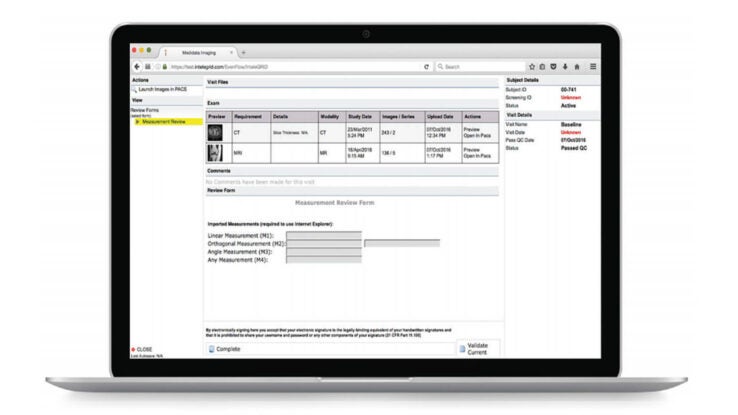
Improve Image and Data Quality
Configured to perform edit checks and de-identification during the image upload process, Rave Imaging’s intelligent workflows simplify image and data collection. The system automates the distribution and review process after upload, according to each protocol.
Related Solutions

Rave EDC
The cornerstone of the Medidata Clinical Cloud™, Rave EDC connects processes, eliminates data reconciliation, and delivers cross-functional and cross-study data insights. When integrated with Imaging, CROs can provide access to all data in one location directly within Rave EDC.

Unified Clinical Data Capture and Management
Rave EDC is at the heart of Medidata’s unified solution for Clinical Data Capture and Management, enabling aggregation and reconciliation of data from multiple sources—Medidata eConsent, Medidata eCOA, MyMedidata, Rave RTSM, Rave Imaging, and Sensor Cloud—and intelligent data review and analysis with Rave TSDV and Medidata Detect.

Rave eCOA
A $2 Billion Opportunity for CROs.
Contact our imaging and EDC experts to learn how Rave Imaging enables CROs to regain control and own imaging revenue across their clinical trials.